Disorders of the Diaphragm in COPD Patients
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory lung disease that causes obstructed airflow from the lungs. Symptoms include breathing difficulty, cough, mucus (sputum) production and wheezing.1 A chest X-Ray image of COPD patients may reveal enlarged lungs, a flattened diaphragm, or potentially dangerous air pockets in the lungs. The diaphragm may appear flattened in the chest as a result of hyperinflation as the lungs push against the diaphragm forcing it downward.1
Chronic airflow limitation imposes a load on respiratory muscles as does lung hyperinflation, flattening the diaphragm and reducing its ability to generate tension. Changes in chest wall geometry and diaphragm position are the most recognized and studied mechanisms contributing to respiratory muscle dysfunction.1 Therefore, it is relevant that respiratory muscles and diaphragm function be assessed in COPD patients. COPD patients often develop hyperinflation. Hyperinflation occurs due to expiratory flow limitation caused by reduced lungs’ elastic recoil and increased airway resistance. Hyperinflation increases during exercise and acute exacerbation. Hyperinflation has a significant negative impact on respiratory muscles, particularly the diaphragm.2
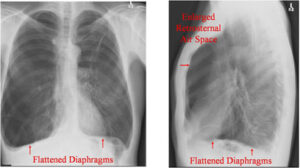
Hyperinflation of the lungs, flattened diaphragm, increased retrosternal air space and hyperlucency of the lungs
The diaphragm is a major respiratory muscle therefore alterations in its structure and function in stable COPD as well as during exacerbations could have significant adverse clinical consequences. Most studies dealing with factors contributing to inspiratory muscle weakness in COPD have focused on the diaphragm, mainly because the diaphragm is the principle muscle of inspiration.3
Diseases presenting with chronic lung hyperinflation such as COPD are another frequent cause of abnormal diaphragm contractility. In these patients, the pressure-generating capacity of the diaphragm is reduced mainly due to lung hyperinflation, which shortens its muscle fibers and places the diaphragm at a mechanical disadvantage by having it contract on the steep part of its length-tension curve.4 Research shows that weak diaphragm muscles can worsen COPD, potentially leading to exacerbations.4
In the presence of diaphragm dysfunction, compensatory responses do not preserves cough, another expulsive behavior requiring near-maximal recruitment of inspiratory muscles. Therefore, the inability to generate normal inspiratory pressures can impair airway clearance and predisposes individuals to pulmonary infections.5
Diaphragm abnormalities contribute to key aspects of pulmonary pathophysiology including: i) impaired airway clearance and predisposition to pneumonia; ii) inability to sustain ventilation during physical activity; iii) shallow breathing pattern that limits alveolar ventilation and gas exchange.5 Finding the triggers for diaphragmatic dysfunction in COPD is especially challenging because several demographic, clinical and laboratory variables such as age, duration of illness, smoking habits, degree of hypoxemia and electrolyte disturbance and structural and functional diaphragmatic abnormalities may be present early in the course of the disease.3 This information may help in improving the quality of life and decrease morbidity and mortality among patients with COPD.
Diaphragm wasting appears to play an important role in compromising diaphragm contractile performance in patients with COPD. The presence of ‘a ceiling’ may render patients with COPD more vulnerable to increase in load during acute exacerbations.6 The increased susceptibility of the COPD diaphragm to inspiratory loading–induced sarcomeric injury appears to be an important finding because it suggests that, during exacerbations of COPD, when patients face acute diaphragm loading, sarcomeres may develop additional injury.7 This could further compromise diaphragm function and consequently result in respiratory failure.
It has been demonstrated that patients with severe COPD generate less maximal inspiratory pressure and transdiaphragmatic pressure by voluntary maneuvers, but also by phrenic nerve stimulation (ruling out effects of central drive), compared to patients without COPD. Therefore, strength is regarded as a limiting factor in diaphragm performance in COPD.3 Considering the clinical relevance of inspiratory muscle weakness in patients with COPD, counteracting diaphragm weakness is of major importance.
A recent study indicates that central impairment occurs early in stable COPD. This is important for predicting disease outcome and emphasizes the importance of early therapeutic interventions before the occurrence of manifest ventilatory insufficiency.8 These findings also have therapeutic implications as it has been seen that in hospitalized patients with acute exacerbation of COPD subjected to noninvasive ventilation (NIV), severe diaphragmatic dysfunction as measured by diaphragmatic ultrasound was seen in almost 25% of patients. Diaphragmatic dysfunction was also associated with NIV failure and short-term mortality in these patients.8
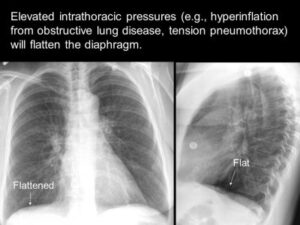
Cough is an effective method of clearing secretions from the larger airways in healthy individuals. However, respiratory muscle impairment leads to difficulty with coughing and clearing secretion, subsequent mucous plugging and pulmonary infection.9 With narrow airways, coughing can have detrimental effects if used inappropriately over an extended period as the primary method of clearing secretions.
Effective mucus clearance is essential for lung health, and airway disease is a consistent consequence of poor clearance.10 Airway clearance techniques (ACTs) to assist with secretion clearance are widely recommended and can include both mucus-mobilizing techniques and assisted cough techniques.10 Studies recommend that ACTs be used as the primary method of mobilizing secretions from the middle and small airways to the larger airways. Then, effective coughing can be used to clear secretions from the larger airways, thereby preserving the integrity of the larger airways.10
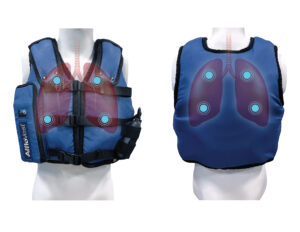
High-frequency chest wall oscillation (HFCWO) airway clearance therapy applies pressure to the chest wall that is accompanied by high-frequency vibration to loosen and mobilize mucus. A recent retrospective study showed that patients with COPD and without bronchiectasis showed changes in hospitalization rate as well as self-reported outcomes of respiratory health and ability to clear secretions with the use of high frequency chest wall oscillation.11 According the study, HFCWO vests designed for treatment of cystic fibrosis, appeared to reduce COPD exacerbations, according to a retrospective study reported here. After 24 months of wearing the vest, patients achieved a 54.4% reduction in the annualized hospitalization rate for respiratory causes.11
AffloVest Mobile Mechanical HFCWO vest therapy was specifically engineered to mimic the gold standard of manual chest PT, streamline therapy, enhance airway clearance, help mobilize lung secretions, and promote treatment adherence. With no bulky hoses and generators as found in other HFCWO therapies, the AffloVest can be utilized in any postural position (I.e. laying down, standing, sitting, inclined, reclined etc) to help improve secretion mobilization. Recent studies have shown that moving patients into different positions affects ventilation in two different ways. First, a change in body position alters regional ventilation as noted above. Second, by increasing the mobility of a patient, oxygen demand increases, resulting in a corresponding increase in minute ventilation and lung volumes. The resultant increase in ventilation allows air to move into obstructed lung units by interdependence and collateral ventilation.10
COPD patients can be prescribed AffloVest Mobile HFCWO therapy with the diagnosis of Disorder of the Diaphragm and thorough chart notes indicating that other treatments aimed at mobilizing secretions have been tried and failed or thorough documentation of why other treatments would not be sufficient or are not an option for a specific patient. Below is an example of a recent chart note for a COPD patient diagnosed with diaphragm dysfunction that was successfully submitted for the prescription of a HFCWO therapy vest.
“Patient has J98.6 Disorders of the Diaphragm. This was documented on the chest XRAY preformed on 10/09/2019. The Radiologist noted “The remainder of the lungs reveal severe emphysematous changes. The diaphragms are flattened”. As a result, the patients cough and deep breathing have been compromised, resulting in recurring pneumonia. The patient has tried the AeroBika and deep breathing/huff coughing. That alone was not able to adequately mobilize mucus and reduce recurring pneumonia. Patient would benefit from HFCWO vest therapy.”
References:
1. Alana Biggers, MD, MPH Does COPD show up on an X-ray? https://www.medicalnewstoday.com/articles/323363
2. Sarkar M, Bhardwaz R, Madabhavi I, Modi M. Physical signs in patients with chronic obstructive pulmonary disease. Lung India. 2019;36(1):38–47.
3. Ottenheijm, C.A., Heunks, L.M. & Dekhuijzen, R.P. Diaphragm adaptations in patients with COPD. Respir Res 9, 12 (2008).
4. Dubé, B.-P.; Dres, M. Diaphragm Dysfunction: Diagnostic Approaches and Management Strategies. J. Clin. Med. 2016, 5, 113.
5. Kelley RC, Ferreira LF. Diaphragm abnormalities in heart failure and aging: mechanisms and integration of cardiovascular and respiratory pathophysiology. Heart Fail Rev. 2017;22(2):191–207.
6. Orozco-Levi M. Structure and function of the respiratory muscles in patients with COPD: impairment or adaptation? Eur Respir J Suppl. 2003; 46: 41-51
7. Ottenheijm CAC, Heunks LMA, Sieck GC, Zhan WZ, Jansen SM, Degens H, de Boo T, Dekhuijzen PNR. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;172:200–205.
8. Antenora F, et al. Prevalence and outcomes of diaphragmatic dysfunction assessed by ultrasound technology during acute exacerbation of COPD: A pilot study. Respirology. 2017;22(2):338–344
9. Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363(23):2233–2247.
10. McIlwaine M, Bradley J, Elborn JS, et al. Personalizing airway clearance in chronic lung disease. Eur Respir Rev 2017; 26: 16008
11. Hansen, Gary, et al Outcomes of high frequency chest wall oscillation (HFCWO) in COPD patients without bronchiectasis. CHEST, Volume 156, Issue 4, A1170